Global pharmaceutical distribution networks and supply chains have become more complex and interconnected, quickening the pace of regulations that mandate pharmaceutical serialization and traceability. As pharmaceutical serialization gains momentum around the globe, it is important to understand the building blocks of traceability systems and how serialization data can be leveraged across the supply chain. Understanding serialization and the ways in which serialized data should be used is of primary importance.
Serialization is the process by which products are marked with a unique identifier—typically a unique number or alphanumeric code—and is beginning to be leveraged to enhance supply chain security. The unique serial number, along with other related information, is typically encoded in a barcode that can be read electronically. Serialization of pharmaceuticals (i.e., simply applying a unique identifier) itself provides virtually no benefit to the supply chain. Rather, it is the use of that serialized data in some manner that enhances supply chain security. This complementary use of the serialized data is commonly referred to as traceability.
Serialization of pharmaceuticals involves assigning a unique identifier to a product for identification purposes, and also capturing that data in a barcode. While serialization of products is leveraged to promote supply chain security, serialization alone has no benefit for the supply chain. The traceability systems that use serialized data to verify, authenticate, track, or trace products in the supply chain are what truly help to secure the supply chain. For serialization to have the optimal benefit, countries should analyze the issues that they would like to address within their market and implement the most efficient method to use serialized data using global standards to be communicated clearly between both companies and countries to identify anomalies before that product is dispensed to the patient.
Serialization means that each individual package is assigned and marked with a standardized unique identifier that can be read and communicated across the supply chain. Though a straightforward concept, the process of serialization is much more complex than setting up packing lines with bar code printers and scanners. In addition to serial number and identification, serialization must include a process for:
Identification
A serial number is a code assigned to an individual item for its lifetime. To identify a unique individual item, serial numbers must be augmented with encoded product identifers. These encoded identifiers must be affixed to all products as they enter the supply chain. GS1, a global standards setting organization, has identified the standards below for identification of health care products. All of the identification standards listed are globally unique identifiers that may be utilized around the world.

[Image Credit: GS1]
Given the unique safety concerns associated with counterfeit, diverted, stolen, and adulterated medications, the inclusion of the expiration date helps to prevent outdated product from being dispensed to patients. Furthermore, the association of expiration date and batch number with a serial number helps to distinguish between trade items with similar production and usage characteristics. Once a GTIN is assigned to a product, this information is uploaded to a live database. All of a company’s entities and trading partners should be able to recognize a GTIN.
As a product moves through the supply chain, additional data elements can provide more specific information about a product. These data elements include:
Capture
The second component of serialization is the method of capturing serialized data (GTIN, expiration date, and batch). For efficiency, all serialized data must be encoded in a barcode that can then be read electronically and recognized further down the supply chain. While GS1 has endorsed several methods of data capture (see below), industry stakeholders have coalesced around the use of a 2D Data Matrix barcode for identification of individual packages of product. Cases or logistic units are typically encoded with a 2D Data Matrix or a linear barcode (e.g., GS1-128).
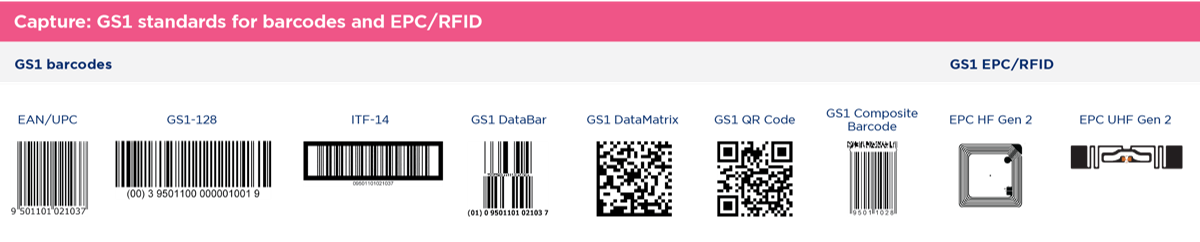
[Image Credit: GS1]
Breaches of the pharmaceutical supply chain can have serious public health consequences. The market for counterfeit medicines has expanded in recent years and increased globalization of trade. Counterfeit, medications pose a risk to patients as they are often substandard products that are physically indistinguishable from valid product. These counterfeit drugs may be inactive or incorrect, or contain improper dosages that are less, or more, potent than the legitimate drug.
The risk of medication diversion and theft is an additional supply chain security concern. Drug diversion is the transfer of any legally prescribed drug from the person for whom it was prescribed to any other person for illicit use, and includes the trading of drugs not legally available for sale in a given country. When drugs are diverted or stolen, the quality and safety of the product cannot be assured or maintained. These drugs may be intentionally altered or damaged due to improper storage or exposure to contaminants. If reintroduced to the market, these products may be ineffective, or may pose a risk of additional health complications for patients.
Traceability systems, which use the serialized data on each package, can authenticate product and/or trace the product’s path through the supply chain. Although serialization requires large investments of time and resources, with the appropriate infrastructure and technology, the data acquired from serialized product can be leveraged to address challenges, such as counterfeiting or diversion, faced by a country.
There are three main levels of packaging for pharmaceutical products moving through the supply chain. Each level has its own serialization requirements based on the contents of the package.
Primary level—the primary level of packaging is the first level. This package is in direct contact with the product. Examples include a medicine strip, vial, single therapy kit, or ampule. Industry consensus is that products should not be serialized at the primary level for cost, security, and logistical reasons. In many cases, the 2D matrix is not able to fit on primary packages, which are too small.
Secondary level—the secondary level is often the smallest level intended to be sold to the dispenser/pharmacy. This may be a package containing one or more primary packages, or a group of primary packages containing a single item. Industry consensus is that the secondary package is the smallest level of packaging that should be serialized.
Tertiary level—the tertiary package is the logistic unit, as described above. Tertiary packages are the shipper, carton, case, pallet, or tote that contains one or more primary/secondary levels of packaging. As noted, tertiary packages are serialized using a SSCC.
The true value of serialization lies in the use of serialization data to trace a specific product (unique identifier) through the supply chain or to validate that the product given to the patient is authentic and undamaged. As depicted in the flow chart below, there are two general approaches and four models for the use of serialized data.
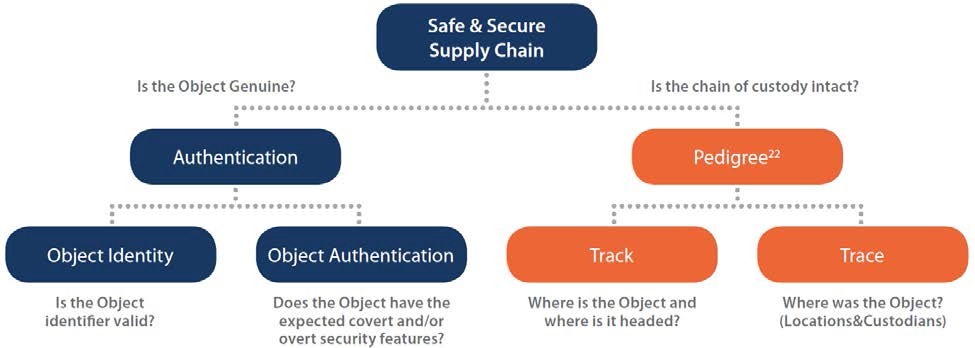
[Image Credit: GS1]
Object Identity (Verification)
In an authentication model, supply chain stakeholders verify that the serial numbers used to identify a particular product were, in fact, affixed to the package by the manufacturer of that product. This is accomplished by cross-checking the product identifiers on a package with the database of existing serial numbers previously populated by the manufacturer and associating them to lot number and expiry date in the manufacturer’s database. Additional indicators of product identity are the integrity of the product packaging and the presence of the brand insignia on the package.
Object Authentication
Serialization is implemented on product packaging, and not the product itself, and therefore verification of serialized data is necessary, but not necessarily exhaustive, to determine the authenticity of the product within the package. Once a product identifier is determined to be valid, an Authentication Model also includes a process for object authentication. While serial numbers play a role in determining the authenticity of a product, a check of the serialized data may be supplemented by checks of both overt and covert security features used for brand protection by manufacturers (e.g., tamper-evident packaging).
Track & Trace
A drug track and trace system is intended to provide, in electronic form, the transaction history of a product including all changes of ownership of a given product. A query of an individual package outlines the movement of a given shipment throughout the supply chain from sale by a manufacturer, though acquisition(s) and sale(s) by one or more wholesalers, manufacturers, or pharmacies, until final sale to a pharmacy or other person furnishing, administering, or dispensing the drug.
Track
Tracking pharmaceuticals allows each stakeholder to identify the current owner of the product and the pathway the product must take to get to its current location.
Trace
Tracing pharmaceuticals means recreating the path of a product, from the manufacturer to the current entity/owner. Tracing systems identify where the product has been and which entities have had ownership of the product.
The implementation of a traceability system effectively requires the interoperable exchange of data. Stakeholders must have servers that can communicate with one another, and data must be transferable, without alteration or translation, from trading partner to trading partner. Global standards for data exchange allow products to be seamlessly followed across country borders and allow for global supply chain security. Utilizing data standards creates a common language among different servers which enables supply chain members to exchange data in a common and understandable format. System efficiencies and cost savings are created when trading partners adhere to standards for data exchange.
Data Sharing
A widely utilized standard for sharing event data is the Electronic Product Code Information Services (EPCIS) standard created by GS1. The goal of EPCIS is to enable disparate applications to create and share data between trading partners and internally about each product event (e.g., the initial serialization or “commissioning” of a product, the shipment of a product, the dispensing of a product). EPCIS can operate within diverse information technology (IT) environments to identify where products are and why they are there.